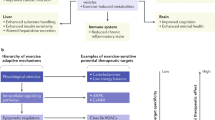
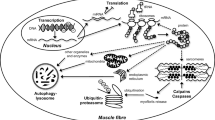
Abstract
Physical activity and sports play major roles in the overall health status of humans. It is well known that regular exercise helps to lower the risk for a broad variety of health problems, such as cardiovascular disease, type 2 diabetes, and cancer. Being physically active induces a wide variety of molecular adaptations, for example fiber type switches or other metabolic alterations, in skeletal muscle tissue. These adaptations are based on exercise-induced changes to the skeletal muscle transcriptome. Understanding their nature is crucial to improve the development of exercise-based therapeutic strategies. Recent research indicates that specifically epigenetic mechanisms, i.e., pathways that induce changes in gene expression patterns without altering the DNA base sequence, might play a major role in controlling skeletal muscle transcriptional patterns. Epigenetic mechanisms include DNA and histone modifications, as well as expression of specific microRNAs. They can be modulated by environmental factors or external stimuli, such as exercise, and eventually induce specific and fine-tuned changes to the transcriptional response. In this review, we highlight current knowledge on epigenetic changes induced in exercising skeletal muscle, their target genes, and resulting phenotypic changes. In addition, we raise the question of whether epigenetic modifications might serve as markers for the design and management of optimized and individualized training protocols, as prognostic tools to predict training adaptation, or even as targets for the design of “exercise mimics”.



Similar content being viewed by others
References
Hechanova RL, Wegler JL, Forest CP. Exercise: a vitally important prescription. JAAPA. 2017;30:17–22.
Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12.
Hoppeler H. Molecular networks in skeletal muscle plasticity. J Exp Biol. 2016;219:205–13.
Sarzynski MA, Ghosh S, Bouchard C. Genomic and transcriptomic predictors of response levels to endurance exercise training. J Physiol. 2017;595:2931–9.
Ntanasis-Stathopoulos J, Tzanninis JG, Philippou A, Koutsilieris M. Epigenetic regulation on gene expression induced by physical exercise. J Musculoskelet Neuronal Interact. 2013;13:133–46.
Ehlert T, Simon P, Moser DA. Epigenetics in sports. Sports Med. 2013;4:93–110.
Pareja-Galeano H, Sanchis-Gomar F, García-Giménez JL. Physical exercise and epigenetic modulation: elucidating intricate mechanisms. Sports Med. 2014;44:429–36.
Soci UPR, Melo SFS, Gomes JLP, Silveira AC, Nóbrega C, de Oliveira EM. Exercise training and epigenetic regulation: multilevel modification and regulation of gene expression. Adv Exp Med Biol. 2017;1000:281–322.
Ecker S, Pancaldi V, Valencia A, Beck S, Paul DS. Epigenetic and transcriptional variability shape phenotypic plasticity. Bioessays. 2018;40(2):1700148.
McGee SL, Walder KR. Exercise and the skeletal muscle epigenome. Cold Spring Harb Perspect Med. 2017;7(9):a029876. https://doi.org/10.1101/cshperspect.a029876.
Meier K, Recillas-Targa F. New insights on the role of DNA methylation from a global view. Front Biosci (Landmark Ed). 2017;22:644–68.
Yong W-S, Hsu F-M, Chen P-Y. Profiling genome-wide DNA methylation. Epigenetics Chromatin. 2016;9:26.
Delatte B, Deplus R, Fuks F. Playing TETris with DNA modifications. EMBO J. 2014;33:1198–211.
Ling C, Rönn T. Epigenetic adaptation to regular exercise in humans. Drug Discov Today. 2014;19:1015–8.
Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15:405–11.
Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson E, et al. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61:3322–32.
Kanzleiter T, Jähnert M, Schulze G, Selbig J, Hallahan N, Schwenk RW, et al. Exercise training alters DNA methylation patterns in genes related to muscle growth and differentiation in mice. Am J Physiol Endocrinol Metab. 2015;308(10):E912–20.
Lindholm ME, Marabita F, Gomez-Cabrero D, Rundqvist H, Ekström TJ, Tegnér J, et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics. 2014;9:1557–69.
Rowlands DS, Page RA, Sukala WR, Giri M, Ghimbovschi SD, Hayat I, et al. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in type 2 diabetic obesity. Physiol Genom. 2014;46:747–65.
Pattamaprapanont P, Garde C, Fabre O, Barrès R. Muscle contraction induces acute hydroxymethylation of the exercise-responsive gene Nr4a3. Front Endocrinol (Lausanne). 2016;7:165. https://doi.org/10.3389/fendo.2016.00165 (eCollection 2016).
King-Himmelreich TS, Schramm S, Wolters MC, Schmetzer J, Möser CV, Knothe C, et al. The impact of endurance exercise on global and AMPK gene-specific DNA methylation. Biochem Biophys Res Commun. 2016;474:284–90.
Lane SC, Camera DM, Lassiter DG, Areta JL, Bird SR, Yeo WK, et al. Effects of sleeping with reduced carbohydrate availability on acute training responses. J Appl Physiol (1985). 2015;119(6):643–55.
Laker RC, Lillard TS, Okutsu M, Zhang M, Hoehn KL, Connelly JJ, et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes. 2014;63:1605–11.
Lochmann TL, Thomas RR, Bennett JP Jr, Taylor SM. Epigenetic modifications of the PGC-1α promoter during exercise induced expression in mice. PLoS One. 2015;10(6):e0129647.
Kasch J, Kanzleiter I, Saussenthaler S, Schürmann A, Keijer J, van Schothorst E, et al. Insulin sensitivity linked skeletal muscle Nr4a1 DNA methylation is programmed by the maternal diet and modulated by voluntary exercise in mice. J Nutr Biochem. 2018;57:86–92.
Nguyen A, Duquette N, Mamarbachi M, Thorin E. Epigenetic regulatory effect of exercise on glutathione peroxidase 1 expression in the skeletal muscle of severely dyslipidemic mice. PLoS One. 2016;11(3):e0151526.
Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, Someren KAV, et al. Methylome of human skeletal muscle after acute and chronic resistance exercise training, detraining and retraining. Sci Data. 2018;5:180213.
Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, van Someren KA, et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep. 2018;8:1898.
Laker RC, Garde C, Camera DM, Smiles WJ, Zierath JR, Hawley JA, et al. Transcriptomic and epigenetic responses to short-term nutrient-exercise stress in humans. Sci Rep. 2017;7:15134.
Brown WM. Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: a bioinformatics meta-analysis. Br J Sports Med. 2015;49:1567–78.
Carter HN, Pauly M, Tryon LD, Hood DA. Effect of contractile activity on PGC-1α transcription in young and aged skeletal muscle. J Appl Physiol. 1985;2018(124):1605–15.
Fisher AG, Seaborne RA, Hughes TM, Gutteridge A, Stewart C, Coulson JM, et al. Transcriptomic and epigenetic regulation of disuse atrophy and the return to activity in skeletal muscle. FASEB J. 2017;31:5268–82.
Stephens NA, Brouwers B, Eroshkin AM, Yi F, Cornnell HH, Meyer C, et al. Exercise response variations in skeletal muscle PCr recovery rate and insulin sensitivity relate to muscle epigenomic profiles in individuals with type 2 diabetes. Diabetes Care. 2018;41:2245–54.
Bajpeyi S, Covington JD, Taylor EM, Stewart LK, Galgani JE, Henagan TM. Skeletal muscle PGC1α -1 nucleosome position and -260 nt DNA methylation determine exercise response and prevent ectopic lipid accumulation in men. Endocrinology. 2017;158(7):2190–9.
Terruzzi I, Senesi P, Montesano A, La Torre A, Alberti G, Benedini S, et al. Genetic polymorphisms of the enzymes involved in DNA methylation and synthesis in elite athletes. Physiol Genom. 2011;43:965–73.
Gates LA, Foulds CE, O’Malley BW. Histone marks in the ‘driver’s seat’: functional roles in steering the transcription cycle. Trends Biochem Sci. 2017;42:977–89.
McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol. 2009;587:5951–8.
McGee SL, Hargreaves M. Histone modifications and exercise adaptations. J Appl Physiol. 1985;2011(110):258–63.
Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Investig. 2007;117:2459–67.
Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab. 2008;295:E698–704.
Joseph JS, Ayeleso AO, Mukwevho E. Exercise increases hyper-acetylation of histones on the Cis-element of NRF-1 binding to the Mef2a promoter: implications on type 2 diabetes. Biochem Biophys Res Commun. 2017;486:83–7.
Hong S, Zhou W, Fang B, Lu W, Loro E, Damle M, et al. Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat Med. 2017;23:223–34.
Ohsawa I, Konno R, Masuzawa R, Kawano F. Amount of daily exercise is an essential stimulation to alter the epigenome of skeletal muscle in rats. J Appl Physiol (1985). 2018;125(4):1097–104.
Barreiro E, Sznajder JI. Epigenetic regulation of muscle phenotype and adaptation: a potential role in COPD muscle dysfunction. J Appl Physiol. 1985;2013(114):1263–72.
Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genom. 2013;45:499–507.
Masuzawa R, Konno R, Ohsawa I, Watanabe A, Kawano F. Muscle type-specific RNA polymerase II recruitment during PGC-1α gene transcription after acute exercise in adult rats. J Appl Physiol (1985). 2018. https://doi.org/10.1152/japplphysiol.00202.2018.
Pandorf CE, Haddad F, Wright C, Bodell PW, Baldwin KM. Differential epigenetic modifications of histones at the myosin heavy chain genes in fast and slow skeletal muscle fibers and in response to muscle unloading. Am J Physiol Cell Physiol. 2009;297:C6–16.
Kawano F, Nimura K, Ishino S, Nakai N, Nakata K, Ohira Y. Differences in histone modifications between slow- and fast-twitch muscle of adult rats and following overload, denervation, or valproic acid administration. J Appl Physiol. 1985;2015(119):1042–52.
Willkomm L, Gehlert S, Jacko D, Schiffer T, Bloch W. p38 MAPK activation and H3K4 trimethylation is decreased by lactate in vitro and high intensity resistance training in human skeletal muscle. PLoS One. 2017;12(5):e0176609.
Ribich S, Harvey D, Copeland RA. Drug discovery and chemical biology of cancer epigenetics. Cell Chem Biol. 2017;24:1120–47.
Penna F, Costelli P. New developments in investigational HDAC inhibitors for the potential multimodal treatment of cachexia. Expert Opin Investig Drugs. 2018. https://doi.org/10.1080/13543784.2019.1557634 (Epub ahead of print).
Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–4.
Polakovičová M, Musil P, Laczo E, Hamar D, Kyselovič J. Circulating microRNAs as potential biomarkers of exercise response. Int J Mol Sci. 2016;17(10):E1553.
Silva GJJ, Bye A, El Azzouzi H, Wisløff U. MicroRNAs as important regulators of exercise adaptation. Prog Cardiovasc Dis. 2017;2017(60):130–51.
Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics. Oncol Rep. 2017;37:3–9.
Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, et al. Interplay between miRNAs and human diseases. J Cell Physiol. 2018;233:2007–18.
Daskalakis NP, Provost AC, Hunter RG, Guffanti G. Noncoding RNAs: stress, glucocorticoids, and posttraumatic stress disorder. Biol Psychol. 2018;83:849–65.
McCarthy JJ. The MyomiR network in skeletal muscle plasticity. Exerc Sport Sci Rev. 2011;39:150–4.
Luo W, Nie Q, Zhang X. MicroRNAs involved in skeletal muscle differentiation. J Genet Genom. 2013;40:107–16.
Horak M, Novak J, Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016;410(1):1–13.
Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One. 2009;4(5):e5610.
Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, et al. The microRNA miR-696 regulates PGC-1 alpha in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. 2010;298:E799–806.
Yamamoto H, Morino K, Nishio Y, Ugi S, Yoshizaki T, Kashiwagi A, et al. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am J Physiol Endocrinol Metab. 2012;303:E1419–27.
Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol. 1985;110:46–59.
Nielsen S, Scheele C, Yfanti C, Akerström T, Nielsen AR, Pedersen BK, et al. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588:4029–37.
Russell AP, Lamon S, Boon H, Wada S, Güller I, Brown EL, et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J Physiol. 2013;591:4637–53.
Oikawa S, Lee M, Motohashi N, Maeda S, Akimoto T. An inducible knockout of Dicer in adult mice does not affect endurance exercise-induced muscle adaptation. Am J Physiol Cell Physiol. 2018. https://doi.org/10.1152/ajpcell.00278.2018.
McCarthy JJ, Esser KA. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol. 1985;2007(102):306–13.
Mueller M, Breil FA, Lurman G, Klossner S, Flück M, Billeter R, et al. Different molecular and structural adaptations with eccentric and conventional strength training in elderly men and women. Gerontology. 2011;57:528–38.
Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, et al. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol. 1985;2011(110):309–17.
Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab. 2008;295:E1333–40.
Ringholm S, Biensø RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, et al. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E649–58.
Fyfe JJ, Bishop DJ, Zacharewicz E, Russell AP, Stepto NK. Concurrent exercise incorporating high-intensity interval or continuous training modulates mTORC1 signaling and microRNA expression in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2016;31:R1297–311.
Makarova JA, Maltseva DV, Galatenko VV, Abbasi A, Maximenko DG, Grigoriev AI, et al. Exercise immunology meets miRNAs. Exerc Immunol Rev. 2014;20:135–64.
Mooren FC, Viereck J, Krüger K, Thum T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am J Physiol Heart Circ Physiol. 2014;306(4):H557–63.
Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589:3983–94.
Wardle SL, Bailey ME, Kilikevicius A, Malkova D, Wilson RH, Venckunas T, et al. Plasma microRNA levels differ between endurance and strength athletes. PLoS One. 2015;10(4):e0122107.
Sawada S, Kon M, Wada S, Ushida T, Suzuki K, Akimoto T. Profiling of circulating microRNAs after a bout of acute resistance exercise in humans. PLoS One. 2013;8(7):e70823.
Bye A, Røsjø H, Aspenes ST, Condorelli G, Omland T, Wisløff U. Circulating microRNAs and aerobic fitness—the HUNT-Study. PLoS One. 2013;8(2):e57496.
Nielsen S, Åkerström T, Rinnov A, Yfanti C, Scheele C, Pedersen BK, et al. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS One. 2014;9(2):e87308.
Aoi W, Ichikawa H, Mune K, Tanimura Y, Mizushima K, Naito Y, et al. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol. 2013;4:80.
Uhlemann M, Möbius-Winkler S, Fikenzer S, Adam J, Redlich M, Möhlenkamp S, et al. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur J Prev Cardiol. 2014;21:484–91.
Horak M, Zlamal F, Iliev R, Kucera J, Cacek J, Svobodova L, et al. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS One. 2018;13(1):e0191060. https://doi.org/10.1371/journal.pone.0191060 eCollection 2018.
Håkansson KEJ, Sollie O, Simons KH, Quax PHA, Jensen J, Nossent AY. Circulating Small non-coding RNAs as biomarkers for recovery after exhaustive or repetitive exercise. Front Physiol. 2018;9:1136.
Ramos AE, Lo C, Estephan LE, Tai YY, Tang Y, Zhao J, et al. Specific circulating microRNAs display dose-dependent responses to variable intensity and duration of endurance exercise. Am J Physiol Heart Circ Physiol. 2018;315(2):H273–83.
Denham J, Marques FZ, O’Brien BJ, Charchar FJ. Exercise: putting action into our epigenome. Sports Med. 2014;44:189–209.
Sharples AP, Stewart CE, Seaborne RA. Does skeletal muscle have an ‘epi’-memory? The role of epigenetics in nutritional programming, metabolic disease, aging and exercise. Aging Cell. 2016;15:603–16.
Acknowledgements
The authors thank Thomas Beiter for help with preparation of the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This review was supported by a grant from the intramural graduate school “iReAct” of the University and the University Hospital Tübingen.
Conflict of interest
Manuel Widmann, Andreas Nieß, and Barbara Munz declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Widmann, M., Nieß, A.M. & Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med 49, 509–523 (2019). https://doi.org/10.1007/s40279-019-01070-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-019-01070-4